Imagine you’re cooking a big pot of stew. You throw in your ingredients, let them simmer, stir occasionally, and after a set time, your delicious meal is ready to serve. A batch reactor works in a similar way—but instead of soup, it’s producing chemicals, pharmaceuticals, or even biofuels.
But what is the function of a batch reactor? Why is it such a crucial piece of equipment in industries ranging from food production to high-tech pharmaceuticals?

What Is a Batch Reactor?
A batch reactor is a type of vessel used in chemical processes where reactants are added at the beginning, allowed to react over a specific period, and then removed at the end of the process. Unlike continuous reactors, where materials constantly flow in and out, a batch reactor operates in a closed system, making it ideal for controlled reactions that require precision and flexibility.
Key Characteristics of a Batch Reactor:
- Non-continuous operation – Ingredients are loaded, the reaction takes place, and then the product is removed before starting a new batch.
- Highly controlled environment – Operators can adjust temperature, pressure, and mixing speed to optimize reaction conditions.
- Versatility – Suitable for a wide range of chemical, pharmaceutical, and food production processes.
- Flexible batch sizes – Can handle small-scale laboratory experiments or larger industrial batches.
Batch Reactor vs. Other Types of Reactors
| Feature | Batch Reactor | Continuous Reactor | Semi-Batch Reactor |
|---|---|---|---|
| Operation Type | Discrete batches | Continuous flow | Combination of both |
| Flexibility | High | Low | Medium |
| Scalability | More challenging | Easier | Moderate |
| Control Over Reaction | Precise | Less precise | Moderate |
| Efficiency for Large-Scale Production | Lower | Higher | Depends on process |
Where Are Batch Reactors Used?
Batch reactors are commonly used in:
- Pharmaceuticals – Drug synthesis and vaccine production.
- Food Industry – Fermentation, brewing, and dairy processing.
- Specialty Chemicals – Adhesives, coatings, and flavoring agents.
- Biotechnology – Enzyme and protein production.
In short, if a process requires precision, flexibility, and strict control, a batch reactor is the go-to choice. However, it’s not always the best option for large-scale manufacturing—more on that later.
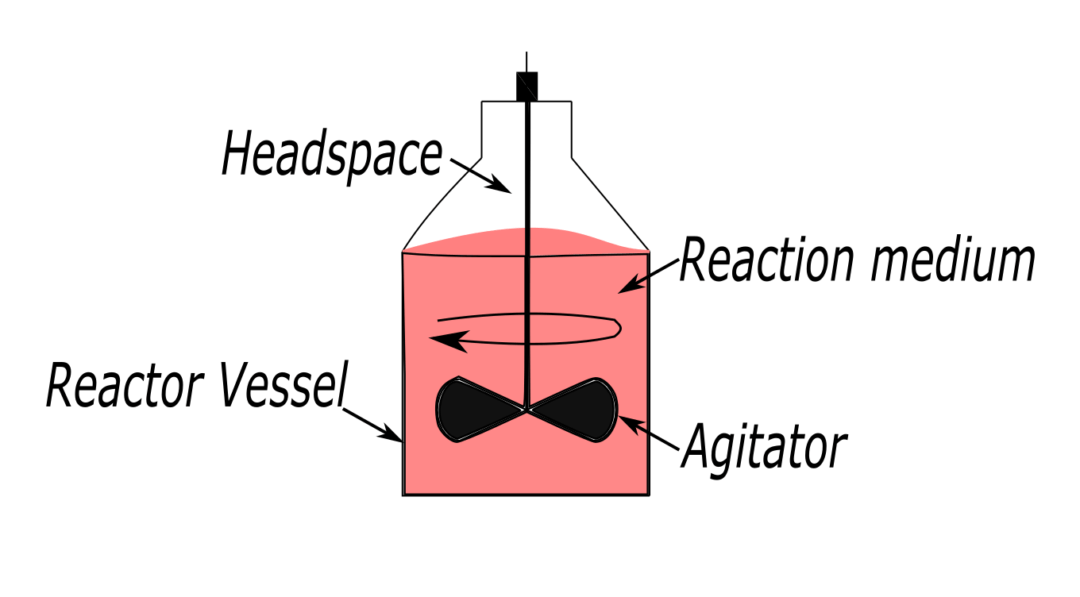
Function of a Batch Reactor
Now, let’s get to the heart of the matter: What is the function of a batch reactor? At its core, a batch reactor is designed to carry out chemical reactions in a controlled environment. It ensures that reactants mix properly, undergo transformation under optimal conditions, and produce the desired product efficiently.
Primary Functions of a Batch Reactor
- Reaction Containment – The reactor provides a sealed, controlled space where chemical reactions can occur without contamination or interference. This is especially critical in pharmaceuticals and high-purity chemical synthesis.
- Mixing and Agitation – Many reactions require thorough mixing to ensure uniformity. A batch reactor is often equipped with mechanical stirrers or impellers to keep the reactants evenly distributed. Poor mixing can lead to inconsistent product quality or incomplete reactions.
- Temperature Control – Chemical reactions can be highly sensitive to temperature. Batch reactors often include:
- Jackets or coils for heating and cooling.
- Temperature probes to monitor conditions in real time.
- Automated systems that adjust temperature as needed.
Example: In pharmaceutical production, maintaining precise temperature control ensures the correct formation of active pharmaceutical ingredients (APIs).
- Pressure Regulation – Some reactions require high-pressure conditions to proceed efficiently. Batch reactors can be designed to operate under vacuum or high-pressure settings, depending on the process.
- Reaction Time Management – Unlike continuous reactors, where reactants are constantly flowing through, batch reactors allow reactions to proceed for a set period. This dwell time can be adjusted to optimize yield and quality.
- Product Separation and Purification – After the reaction is complete, batch reactors can incorporate filtration, settling, or decanting steps to separate the final product from unwanted byproducts or impurities.
- Process Flexibility – A major advantage of batch reactors is their ability to handle multiple types of reactions in the same equipment. Manufacturers can easily switch between different formulations without extensive reconfiguration.
Real-World Example: How a Batch Reactor Works in Drug Production
Let’s take the example of antibiotic synthesis in a pharmaceutical plant:
- Step 1: Loading Reactants – Chemical precursors, solvents, and catalysts are added to the reactor.
- Step 2: Reaction Phase – The reactor maintains specific temperature and pressure conditions while the ingredients react.
- Step 3: Monitoring & Adjustments – Sensors track temperature, pH, and pressure to ensure optimal conditions.
- Step 4: Product Extraction – Once the reaction is complete, the antibiotic compound is extracted and sent for purification.
- Step 5: Reactor Cleaning – Before starting the next batch, the reactor is cleaned and sterilized.
This batch process ensures precise control over the drug’s composition, a critical factor in the pharmaceutical industry.
The function of a batch reactor is to provide a highly controlled environment where chemical reactions can occur efficiently, safely, and with high precision. It’s the preferred choice when flexibility and quality are more important than mass production speed.

Types of Batch Reactors
Not all batch reactors are created equal. Depending on the process, industry, and chemical reaction involved, different types of batch reactors are used to optimize performance. Let’s break down the most common types and their specific applications.
1. Stirred Tank Batch Reactor (STBR)
This is the most common type of batch reactor, featuring a mechanical stirrer to ensure uniform mixing of reactants. It’s widely used in:
- Pharmaceuticals – Drug formulation and active ingredient synthesis.
- Food Processing – Fermentation processes like beer brewing.
- Fine Chemicals – Specialty chemicals and fragrances.
🔹 Example: A pharmaceutical company producing painkillers would use a stirred tank batch reactor to mix chemical precursors thoroughly before crystallizing the final drug compound.
2. Jacketed Batch Reactor
This type of reactor comes with a jacketed outer layer that allows for heating or cooling by circulating fluids (e.g., steam or coolant). It’s used when precise temperature control is necessary.
- Biotechnology – Enzyme production, vaccine synthesis.
- Polymer Manufacturing – Production of specialty plastics and adhesives.
- Chemical Synthesis – Temperature-sensitive reactions requiring controlled heat transfer.
🔹 Example: In polymer production, a jacketed batch reactor ensures that the reaction temperature remains within an optimal range, preventing degradation of the final material.
3. Pressure Batch Reactor
Certain reactions require high-pressure conditions to proceed efficiently. Pressure batch reactors are designed with reinforced walls to handle extreme pressure and prevent leaks.
- Petrochemical Industry – Hydrogenation and hydrocracking reactions.
- Aerospace & Defense – Advanced material synthesis.
- Gas-to-Liquid Processes – Converting gases into liquid fuels or specialty chemicals.
🔹 Example: In the hydrogenation of vegetable oils (used for making margarine), a pressure batch reactor is used to force hydrogen gas into liquid oils under controlled conditions.
4. Vacuum Batch Reactor
Some reactions require a vacuum environment to prevent oxidation, improve purity, or enhance reaction efficiency. These reactors remove air and other gases, creating a low-pressure environment.
- Pharmaceuticals – Sterile drug production.
- Nanotechnology – Advanced material fabrication.
- Organic Chemistry – Vacuum distillation processes.
🔹 Example: In the production of high-purity silicon for semiconductors, a vacuum batch reactor removes contaminants, ensuring the material meets strict electronic industry standards.
5. Specialized Bioreactors
Batch reactors are also extensively used in biotechnology, where they are known as bioreactors. These are designed to cultivate microorganisms, cells, or enzymes in a controlled environment.
- Pharmaceuticals – Vaccine production, insulin synthesis.
- Agriculture – Production of biofertilizers and biopesticides.
- Food Industry – Fermentation for yogurt, cheese, and probiotic drinks.
🔹 Example: In vaccine manufacturing, a bioreactor is used to cultivate bacteria or viruses under sterile conditions before they are harvested for medical use.
Choosing the Right Batch Reactor
| Type of Batch Reactor | Best For | Key Advantage |
|---|---|---|
| Stirred Tank Batch Reactor | General-purpose reactions | Excellent mixing and control |
| Jacketed Batch Reactor | Temperature-sensitive processes | Precise heating & cooling |
| Pressure Batch Reactor | High-pressure reactions | Can handle extreme conditions |
| Vacuum Batch Reactor | Oxygen-sensitive processes | Prevents oxidation & contamination |
| Bioreactor | Cell cultures & fermentation | Supports biological processes |
Choosing the right batch reactor depends on temperature control, pressure requirements, reaction time, and purity needs. While stirred tank reactors dominate general chemical processing, specialized reactors are essential for high-tech industries like pharmaceuticals and materials science.

How Does a Batch Reactor Work?
Now that we know the different types of batch reactors, let’s break down exactly how a batch reactor operates from start to finish. Whether it’s making pharmaceuticals, polymers, or even craft beer, the core process remains the same.
Step-by-Step Process of a Batch Reactor
1. Loading the Reactants
Before the reaction can begin, all necessary materials—reactants, solvents, catalysts—are carefully measured and added to the reactor. This step is crucial because:
- The correct stoichiometric ratio (amount of each reactant) ensures an efficient reaction.
- Certain materials may need pre-mixing or pre-heating before being introduced.
- In cases of hazardous reactions, inert gases like nitrogen may be used to create a safe environment.
🔹 Example: In pharmaceutical manufacturing, an antibiotic precursor must be added in precise amounts to prevent unwanted side reactions.
2. Mixing and Agitation
Once the reactants are inside, the reactor uses mechanical stirrers or impellers to ensure proper mixing. Good mixing is essential because:
- It prevents localized concentration variations, ensuring a uniform reaction.
- It enhances heat and mass transfer, speeding up the process.
- It reduces the formation of byproducts by distributing catalysts and reactants evenly.
🔹 Example: In polymer manufacturing, improper mixing can result in uneven polymerization, leading to defective plastic materials.
3. Controlling Temperature and Pressure
Many reactions require precise temperature and pressure control to maximize yield and prevent unwanted side effects. Batch reactors use:
- Jackets and coils – Circulating hot or cold fluids to adjust temperature.
- Automated sensors – Continuously monitoring and adjusting conditions.
- Pressure control systems – Keeping gas-phase reactions within safe limits.
🔹 Example: In biofuel production, maintaining the right temperature ensures complete conversion of raw materials into usable fuel.
4. Reaction Progress and Monitoring
The reaction is allowed to proceed for a predetermined amount of time, and operators track progress using:
- pH meters – Important in biochemical and food fermentation processes.
- Spectroscopy or chromatography – Used in pharmaceuticals to check compound purity.
- Gas analyzers – Measuring gas evolution in chemical synthesis.
🔹 Example: In vaccine production, constant monitoring is required to ensure that bacteria or viruses grow under optimal conditions.
5. Stopping the Reaction
Once the reaction reaches completion (determined by monitoring product yield or composition), the process must be halted immediately to prevent:
- Overreaction, which could produce unwanted byproducts.
- Loss of product purity.
- Excessive heat generation that could lead to safety hazards.
Methods for stopping the reaction include:
- Cooling down the system to slow reaction rates.
- Adding inhibitors or neutralizers to halt chemical activity.
- Adjusting pressure to shift equilibrium (for gas-phase reactions).
🔹 Example: In polymerization reactions, adding a quenching agent can stop the process and stabilize the final product.
6. Product Extraction and Purification
Once the reaction is complete, the final product must be separated from the reaction mixture. Common separation techniques include:
- Filtration – Removing solid catalysts or byproducts.
- Distillation – Used in chemical and pharmaceutical industries to separate volatile components.
- Centrifugation – Common in biotech applications to extract biological products.
🔹 Example: In perfume manufacturing, distillation is used to extract essential oils from plant materials.
7. Cleaning and Preparation for the Next Batch
Before a new batch can begin, the reactor must be thoroughly cleaned to:
- Prevent contamination (especially critical in food and pharmaceutical industries).
- Ensure consistent product quality.
- Avoid cross-reactions between leftover residues and new reactants.
Methods include:
- Steam cleaning for sterilization.
- Solvent washes to remove chemical residues.
- Automated Cleaning-in-Place (CIP) systems for fast, efficient cleaning.
🔹 Example: In pharmaceutical production, strict regulatory guidelines (such as Good Manufacturing Practices, GMP) require reactors to be fully cleaned and validated before producing a new batch of medicine.
The Batch Reactor Cycle: A Quick Overview
| Step | Purpose | Example |
|---|---|---|
| 1. Loading | Introduce raw materials in precise amounts. | Adding reactants for a new drug synthesis. |
| 2. Mixing | Ensure uniform reaction conditions. | Stirring polymer components to avoid defects. |
| 3. Temperature/Pressure Control | Maintain optimal reaction conditions. | Keeping fermentation at the right temperature. |
| 4. Monitoring | Track reaction progress. | Checking pH levels in vaccine production. |
| 5. Stopping Reaction | Halt chemical changes at the right time. | Using cooling agents in polymerization. |
| 6. Product Extraction | Separate the final product from byproducts. | Distilling essential oils for perfumes. |
| 7. Cleaning | Prepare reactor for the next batch. | Sterilizing equipment for medical-grade products. |
A batch reactor is like a high-tech cooking pot, ensuring that ingredients are mixed, processed, and purified with precision. This controlled approach makes batch reactors ideal for applications where product quality, flexibility, and purity matter more than speed.